Research Outline
Research Outline
We have been interested in specific microfluidic behavior of mixed solution in a microspace. One of the present main research subjects of our group is "Academic Systematization of Separation Analysis Based on Phase Separation Multiphase Flow and Its Practical Technical Improvements", This work is supported by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (MEXT) (No. 17H03083) (2017-2021).
Main Reference Materials
- Investigation of Tube Radial Distribution Phenomenon (TRDP) and Its Function Appearance: Kazuhiko Tsukagoshi, Kimura Keibun-Sha, Chapters 1-14, pp. 1-372,September, 2015.
- Fundamental Research and Application of the Specific Fluidic Behavior of Mixed Solvents in a Microspace (Invited Review): Kazuhiko Tsukagoshi, Analytical Sciences, 30, 65-73 (2014).
- Investigation of Specific Microfluidic Flow with Two-phase Separation Mixed Solvent Solutions and Application to Flow Technology: Kazuhiko Tsukagoshi, Journal of Flow Injection Analysis, 32, 89-95 (2015).
- Consideration of Inner and Outer Phase Configuration in Tube Radial Distribution Phenomenon Based on Viscous Dissipation in a Microfluidic Flow Using Various Types of Mixed Solvent Solutions: Satoshi Fujinaga, Masahiko Hashimoto, Kazuhiko Tsukagoshi, and Jiro Mizushima, Analytical Sciences, 32, 455-461 (2016).
- Consideration of Tube Radial Distribution Phenomenon under Laminar Flow Conditions Based on the Weber Number: Satoshi Fujinaga, Masahiko Hashimoto, Kazuhiko Tsukagoshi, and Jiro Mizushima, Journal of Chemical Engineering of Japan, 48, 947-952 (2015).
- Tube Radial Distribution Chromatography System Developed by Combining Commercially Available HPLC System and Open-Tubular Capillary Tube as Separation Column: Kento Yamada, Hyo Kan, and Kazuhiko Tsukagoshi, Talanta, 183, 89-93 (2018).
- Phase-Separation Multiphase Flow: Preliminary Application to Analytical Chemistry; Kazuhiko Tsukagoshi, Analytical Sciences, 40, 9-28 (2024).
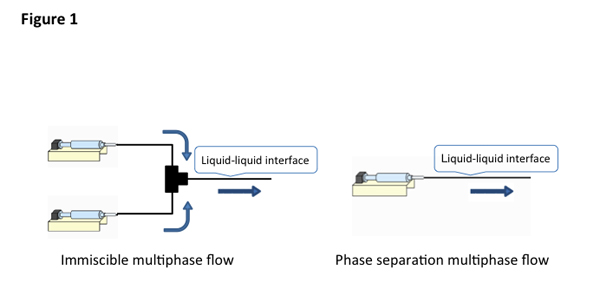
Immiscible multiphase flow vs. phase separation multiphase flow
The conventional multiphase, that is called immiscible multiphase flow, is generated through mixing water and hydrophobic organic solvent solution in the flow, providing kinetic liquid-liquid interface. We have studied a new type of multiphase flow in a microspace that is call "phase separation multiphase flow", in contrast to the immiscible multiphase flow. Typical flow systems for immiscible and phase separation multiphase flow are shown in Figure1.
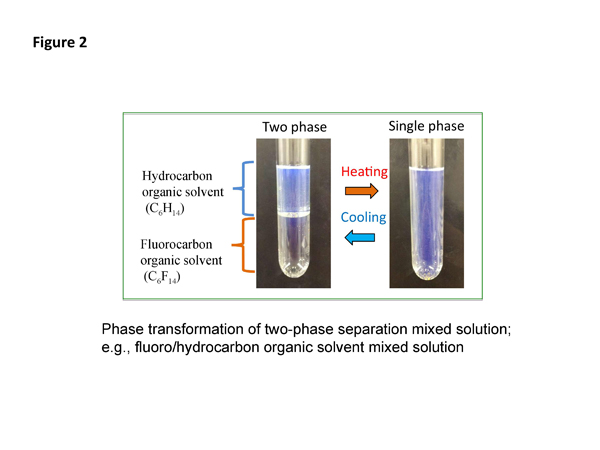
Two-phase separation mixed solutions
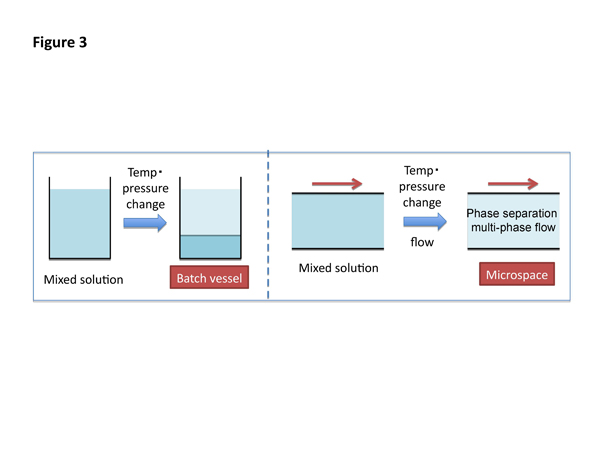
Two-phase separation mixed solutions, such as ternary water/hydrophilic/hydrophobic organic mixed solution, micelle aqueous mixed solution, and ionic liquid aqueous mixed solution, are used in the phase separation multiphase flow. The two-phase separation mixed solutions separate through phase transformation by changing temperature and/or pressure into upper and lower phases in a batch vessel. As an example, phase transformation of fluoro/hydrocarbon organic solvent mixed solution as two-phase separation mixed solution is shown in Figure 2.
Creation of phase separation multiphase flow
While, when the homogeneous two-phase separation mixed solutions are delivered into a microspace under laminar flow conditions changing temperature and/or pressure, the phase transformation occurs in the microspace, leading to phase separation multiphase flow having kinetic liquid-liquid interface, as shown in Figure 3.
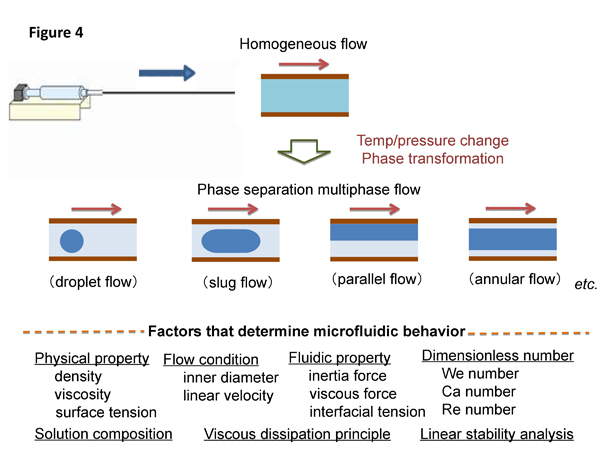
We can observe droplet, slug, parallel, and annular flows under different conditions in phase separation multiphase flow, where there are various factors that determine such microfluidic flows including liquid-liquid interface. Phase separation multiphase flows and the related factors are illustrated in Figure 4.
TRDP and TRDF
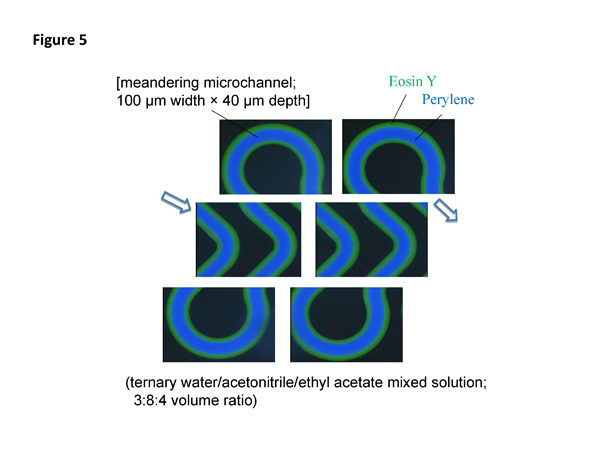
We are especially interested in a stable annular flow having inner and outer phases provided by the phase separation multiphase flow. Such a unique annular flow has not been reported in a microspace having less than several hundred micrometer inner diameter by the conventional immiscible multiphase flow. We call such specific microfluidic behavior and flow "tube radial distribution phenomenon" (TRDP) and "tube radial distribution flow" (TRDF), respectively. We can easily observe TRDF even in meandering microchannel; the fluorescence photograph is shown in Figure 5.
Configuration of inner and outer phase in TRDF
Which solutions separated by phase transformation occupy an inner and outer phase, respectively, in the TRDF? The phase configuration is determined by fluidic energy, minimizing the energy in a microfluidic flow. The phase configuration is theoretically and experimentally discussed with viscous dissipation principle and linear stability analysis. When the difference between the viscosities of the two phases is large, the phase with the higher viscosity forms as the inner phase regardless of the volume ratio in the TRDF, whereas when the difference is small, the phase with the larger volume forms as the inner phase.
Caution !
Some scientists may think that an outer phase in TRDF flow is induced with interaction in chemical characteristics between the inner wall-surface and the solvent molecules. But the inner wall nature of microspace can never induce such mm scale liquid phase through chemical interaction.
TRDC, TRDE, TRDR, and TRDM
A capillary chromatography system, operating under TRDP, where the outer phase functions as a pseudo-stationary phase under laminar flow conditions, has been developed. Such a system is referred to as "tube radial distribution chromatography" (TRDC). We have also applied TRDP for extraction, chemical reaction, and mixing processes. The respective TRDP-based processes are coined as "tube radial distribution extraction" (TRDE), "tube radial distribution reaction" (TRDR), and "tube radial distribution mixing" (TRDM). Since the development of TRDP in 2009, the latter has been widely investigated in several areas pertaining to microfluidics.
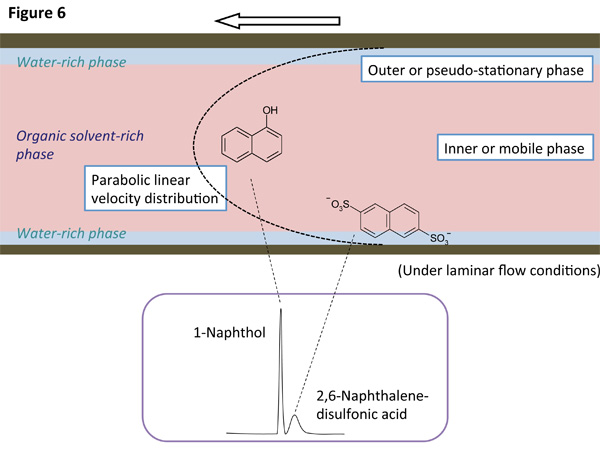
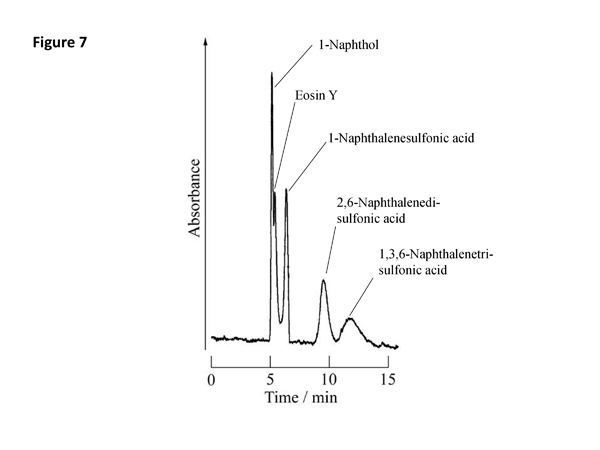
Separation mechanism and chromatogram of TRDC
We have developed capillary chromatography based on TRDF, where the inner and outer phases in an annular flow work as mobile and pseudo-stationary phases in chromatography, respectively. We call a new type of chromatography "tube radial distribution chromatography" (TRDC). In the TRDC analytes distribute between the two phases in a microspace under laminar flow conditions that feature parabolic linear velocity distribution, undergoing chromatographic separation. They are separated just through an open-tubular capillary tube without any specific treatments, such as using packed and monolithic columns as well as applying high voltages. The separation mechanism of the TRDC is illustrated in Figure 6 and the chromatogram obtained for model analytes is also shown in Figure 7.